This website uses cookies. By continuing to browse the site, you are agreeing to our use of cookies
Optimize Data for Meaningful Clinical Trials: Separate Noise from Insights
Life Sciences & Healthcare
October 28, 2020
In the previous blog, we went into the details of cost components of a trial and discussed how latest technologies can help clinical research organizations optimize operations and bring cost efficiency. Let us now look at the Data aspect of clinical trials and how automation can add value by enabling transparency and real-time visibility into clinical trial operations and data.
Data & Trials
In today’s world, data is extremely important in any industry – more so in the clinical trial industry where each trial spans across many years, is cost and effort intensive, and the outcome impacts the wellbeing and life of people. For clinical trials, there is an exponentially increasing volume of data that is collected from different sources, locations and in different formats. Getting all these data in a timely manner and in a harmonized format has been a major challenge.
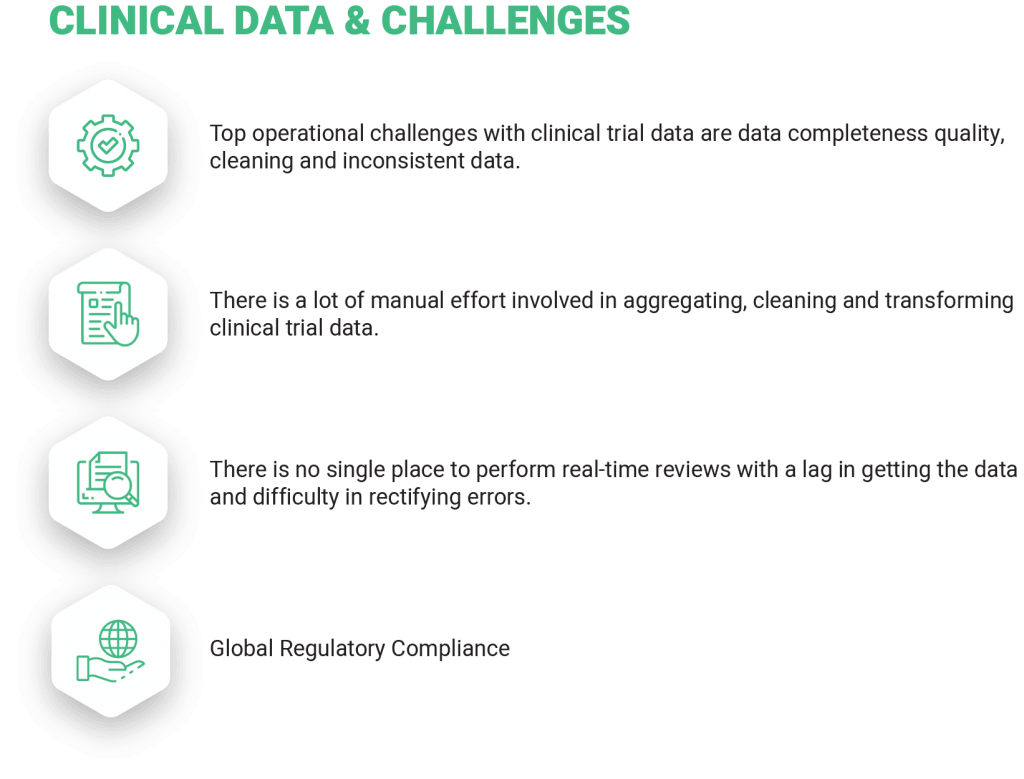
There is an increasing need to provide transparency and real-time visibility into clinical trial operations and data while using the same to drive intelligent business processes. Planning for and running a clinical trial or study is becoming highly complex and requires a Unified Clinical Data Platform aided with advanced statistical analytics and artificial intelligence. An integrated view of various processes is needed to compress time, to deal with problems and adapt to changes proactively.
Towards an Automation-Led Scalable Data Infrastructure
Clinical organizations are undertaking data and analytics modernization journeys to address some of the clinical data challenges. This journey needs to be planned, keeping in mind the challenges of clinical data and assessment of current data infrastructure.
Cloud-based infrastructure is evolving as a viable means to centralize large volumes of clinical data. They are flexible and scalable, and they include real-time open architecture to connect silos of clinical and operational data. Most of the cloud vendors today have a dedicated healthcare cloud which adheres to various regulatory standards. Additionally, there is support for Healthcare APIs – FHIR APIs, HL7 v2 with DICOM images to help create an effective ingestion framework. An efficient ingestion framework that supports both real-time and batch data is extremely important, considering the variety of input clinical data sources.
Data governance is another important aspect that guides the process of integrating multiple, diverse data streams to create a central repository. Automation-led data governance helps in the overall management of availability, usability, integrity and security of data used – this becomes even more critical in a highly regulated industry. The entire transformation and governance process need to be enabled by automation.
Data as a key driver for Digitization
An efficient centralized clinical data fabric greatly enhances the overall trial effectiveness. There is visible, actionable study intelligence that can be used to track startup operations, conduct risk-based clinical monitoring, and enable realistic trial designs. Clinical trials like Insilico trials and adaptive trials based on statistical design, analysis and computer simulations are heavily dependent on comprehensive and high-quality datasets.
The requirements of clinical trials are becoming more complex with the dynamic needs of pharma, biopharma and medical device industries. Intelligent analysis of Real-World Data collected from various sources like claims and provider databases and evidence gathered from them assist in understanding the true patient and epidemiology landscape, design clinical trials based on the findings and refine endpoints. The importance of data will keep on increasing as we move towards addressing more unique health conditions and personalized medicine.
So far, we have looked at the aspects of cost and data optimization by enabling a truly digital clinical trial process. The next blog will cover the challenges of the clinical process and how we can move towards a more digital and efficient clinical enterprise.
Watch this space for the next blog.
Read our first blog in this series titled ‘Reimagine Clinical Trials Optimization in the Digital Era‘ here.
Related Blogs

How Agentic AI is Transforming Pharma Sales: Hexaware’s Agentic AI Solutions
- Life Sciences & Healthcare
- Generative AI

Top Digital Healthcare Trends Shaping 2025 and Beyond
- Life Sciences & Healthcare
- Digital & Software Solutions

The Impact of Generative AI in Healthcare and HCP Engagement
- Life Sciences & Healthcare
- Digital & Software Solutions

Digital Transformation in Pharma: Revolutionizing Patient Care
- Life Sciences & Healthcare
- Digital & Software Solutions

How Digital Healthcare is Shaping Patient-Centric Care
- Life Sciences & Healthcare
- Digital & Software Solutions

CRM in Pharma Industry: How CRM Assessments are Shaping the Future of Pharma Sales
- Life Sciences & Healthcare

How Life Sciences and Healthcare Content Hubs Can Address Your Biggest Content Challenges
- Life Sciences & Healthcare

From Data to Discovery: Generative AI for Research in Life Sciences
- Life Sciences & Healthcare
- Generative AI

Generative AI for Protocol Authoring in Life Sciences
- Life Sciences & Healthcare
- Generative AI

Generative AI in Life Sciences: From Drug Discovery to Personalized Medicine
- Life Sciences & Healthcare
- Generative AI

Ready to Pursue Opportunity?
Every outcome starts with a conversation








